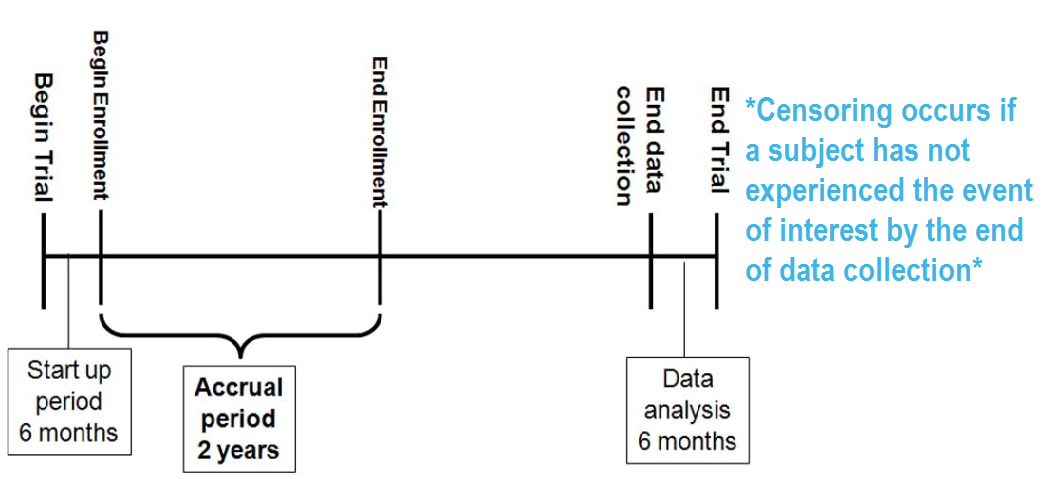
This presentation will cover some basics of survival analysis, and the following series of tutorial papers can be helpful for additional reading:
Clark, T., Bradburn, M., Love, S., & Altman, D. (2003). Survival analysis part I: Basic concepts and first analyses. 232-238. ISSN 0007-0920.
M J Bradburn, T G Clark, S B Love, & D G Altman. (2003). Survival Analysis Part II: Multivariate data analysis – an introduction to concepts and methods. British Journal of Cancer, 89(3), 431-436.
Bradburn, M., Clark, T., Love, S., & Altman, D. (2003). Survival analysis Part III: Multivariate data analysis – choosing a model and assessing its adequacy and fit. 89(4), 605-11.
Clark, T., Bradburn, M., Love, S., & Altman, D. (2003). Survival analysis part IV: Further concepts and methods in survival analysis. 781-786. ISSN 0007-0920.
Time-to-event data are common in many other fields. Some other examples include:
Because time-to-event data are common in many fields, it also goes by names besides survival analysis including:
A key feature of survival data is censoring.

A subject may be censored due to:
Specifically these are examples of right censoring. Left censoring and interval censoring are also possible, and methods exist to analyze these types of data, but this tutorial will be focus on right censoring.
To illustrate the impact of censoring, suppose we have the following data:
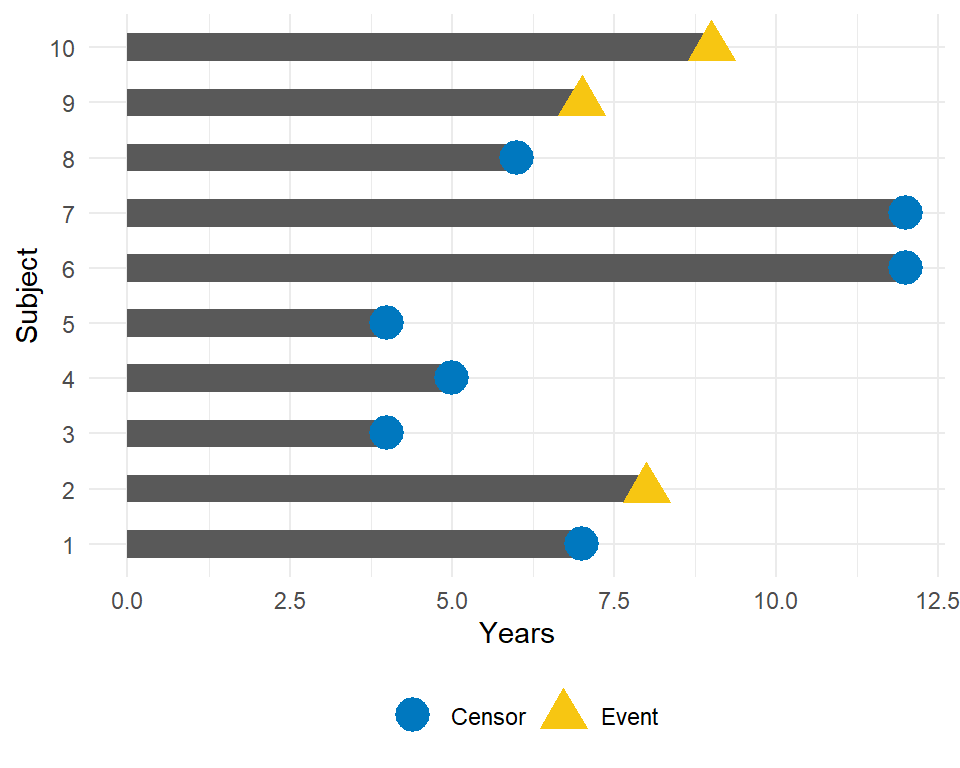
How would we compute the proportion who are event-free at 10 years?
Survival analysis techniques provide a way to appropriately account for censored patients in the analysis.
Other reasons specialized analysis techniques are needed:
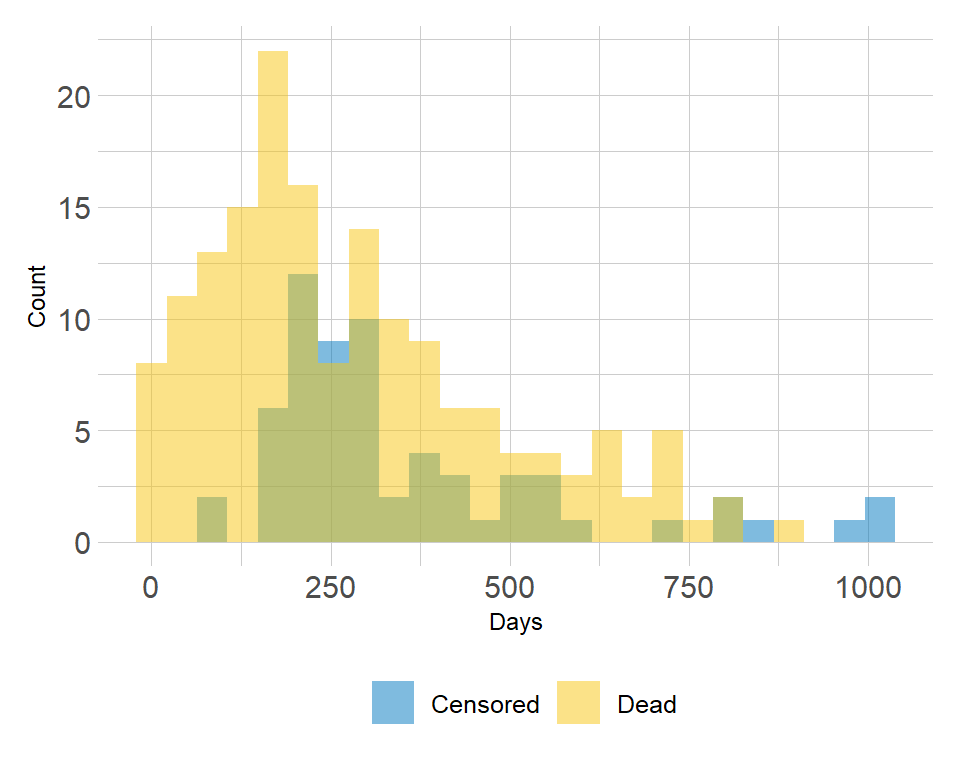
Example of the distribution of follow-up times according to event status:
To analyze survival data, we need to know the observed time \(Y_i\) and the event indicator \(\delta_i\) . For subject \(i\) :
The probability that a subject will survive beyond any given specified time
\[S(t) = Pr(T>t) = 1 - F(t)\]
\(S(t)\) : survival function \(F(t) = Pr(T \leq t)\) : cumulative distribution function
In theory the survival function is smooth; in practice we observe events on a discrete time scale.
The survival probability at a certain time, \(S(t)\) , is a conditional probability of surviving beyond that time, given that an individual has survived just prior to that time. The survival probability can be estimated as the number of patients who are alive without loss to follow-up at that time, divided by the number of patients who were alive just prior to that time.
The Kaplan-Meier estimate of survival probability at a given time is the product of these conditional probabilities up until that given time.
At time 0, the survival probability is 1, i.e. \(S(t_0) = 1\) .
In this section, we will use the following packages:
# install.packages(c("lubridate", "ggsurvfit", "gtsummary", "tidycmprsk")) library(lubridate) library(ggsurvfit) library(gtsummary) library(tidycmprsk) # devtools::install_github("zabore/condsurv") library(condsurv)Throughout this section, we will use the lung dataset from the package as example data. The data contain subjects with advanced lung cancer from the North Central Cancer Treatment Group. We will focus on the following variables throughout this tutorial:
Note that the status is coded in a non-standard way in this dataset. Typically you will see 1=event, 0=censored. Let’s recode it to avoid confusion:
lung % mutate( status = recode(status, `1` = 0, `2` = 1) )Here are the first 6 observations:
head(lung[, c("time", "status", "sex")])## time status sex ## 1 306 1 1 ## 2 455 1 1 ## 3 1010 0 1 ## 4 210 1 1 ## 5 883 1 1 ## 6 1022 0 1Note: the Surv() function in the package accepts by default TRUE/FALSE, where TRUE is event and FALSE is censored; 1/0 where 1 is event and 0 is censored; or 2/1 where 2 is event and 1 is censored. Please take care to ensure the event indicator is properly formatted.
Data will often come with start and end dates rather than pre-calculated survival times. The first step is to make sure these are formatted as dates in R.
Let’s create a small example dataset with variables sx_date for surgery date and last_fup_date for the last follow-up date:
date_ex ## # A tibble: 3 × 2 ## sx_date last_fup_date ## ## 1 2007-06-22 2017-04-15 ## 2 2004-02-13 2018-07-04 ## 3 2010-10-27 2016-10-31We see these are both character variables, but we need them to be formatted as dates.
We will use the package to work with dates. In this case, we need to use the ymd() function to change the format, since the dates are currently in the character format where the year comes first, followed by the month, and followed by the day.
date_ex % mutate( sx_date = ymd(sx_date), last_fup_date = ymd(last_fup_date) ) date_ex## # A tibble: 3 × 2 ## sx_date last_fup_date ## ## 1 2007-06-22 2017-04-15 ## 2 2004-02-13 2018-07-04 ## 3 2010-10-27 2016-10-31Now we see that the two dates are formatted as date rather than as character. Access the help page with ?ymd to see all date format options.
Now that the dates are formatted, we need to calculate the difference between start and end dates in some units, usually months or years. Using the package, the operator %--% designates a time interval, which is then converted to the number of elapsed seconds using as.duration() and finally converted to years by dividing by dyears(1) , which gives the number of seconds in a year.
date_ex % mutate( os_yrs = as.duration(sx_date %--% last_fup_date) / dyears(1) ) date_ex## # A tibble: 3 × 3 ## sx_date last_fup_date os_yrs ## ## 1 2007-06-22 2017-04-15 9.82 ## 2 2004-02-13 2018-07-04 14.4 ## 3 2010-10-27 2016-10-31 6.01Now we have our observed time for use in survival analysis.
Note: we need to load the package using a call to library in order to be able to access the special operators (similar to situation with pipes - i.e. we can’t use lubridate::ymd() and then expect to use the special operators).
The Kaplan-Meier method is the most common way to estimate survival times and probabilities. It is a non-parametric approach that results in a step function, where there is a step down each time an event occurs.
The Surv() function from the package creates a survival object for use as the response in a model formula. There will be one entry for each subject that is the survival time, which is followed by a + if the subject was censored. Let’s look at the first 10 observations:
Surv(lung$time, lung$status)[1:10]## [1] 306 455 1010+ 210 883 1022+ 310 361 218 166We see that subject 1 had an event at time 306 days, subject 2 had an event at time 455 days, subject 3 was censored at time 1010 days, etc.
The survfit() function creates survival curves using the Kaplan-Meier method based on a formula. Let’s generate the overall survival curve for the entire cohort, assign it to object s1 , and look at the structure using str() :
## List of 16 ## $ n : int 228 ## $ time : num [1:186] 5 11 12 13 15 26 30 31 53 54 . ## $ n.risk : num [1:186] 228 227 224 223 221 220 219 218 217 215 . ## $ n.event : num [1:186] 1 3 1 2 1 1 1 1 2 1 . ## $ n.censor : num [1:186] 0 0 0 0 0 0 0 0 0 0 . ## $ surv : num [1:186] 0.996 0.982 0.978 0.969 0.965 . ## $ std.err : num [1:186] 0.0044 0.00885 0.00992 0.01179 0.01263 . ## $ cumhaz : num [1:186] 0.00439 0.0176 0.02207 0.03103 0.03556 . ## $ std.chaz : num [1:186] 0.00439 0.0088 0.00987 0.01173 0.01257 . ## $ type : chr "right" ## $ logse : logi TRUE ## $ conf.int : num 0.95 ## $ conf.type: chr "log" ## $ lower : num [1:186] 0.987 0.966 0.959 0.947 0.941 . ## $ upper : num [1:186] 1 1 0.997 0.992 0.989 . ## $ call : language survfit(formula = Surv(time, status) ~ 1, data = lung) ## - attr(*, "class")= chr "survfit"Some key components of this survfit object that will be used to create survival curves include:
Note: alternatively, survival plots can be created using base R or the package.
The package works best if you create the survfit object using the included ggsurvfit::survfit2() function, which uses the same syntax to what we saw previously with survival::survfit() . The ggsurvfit::survfit2() tracks the environment from the function call, which allows the plot to have better default values for labeling and p-value reporting.
survfit2(Surv(time, status) ~ 1, data = lung) %>% ggsurvfit() + labs( x = "Days", y = "Overall survival probability" )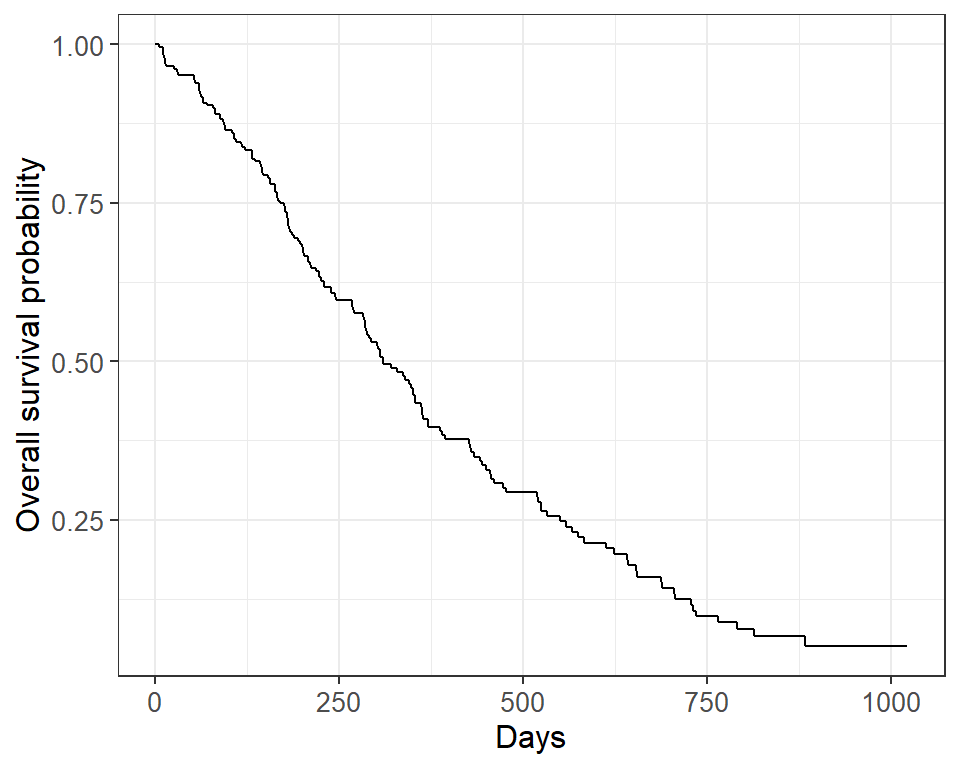
The default plot in ggsurvfit() shows the step function only. We can add the confidence interval using add_confidence_interval() :
survfit2(Surv(time, status) ~ 1, data = lung) %>% ggsurvfit() + labs( x = "Days", y = "Overall survival probability" ) + add_confidence_interval()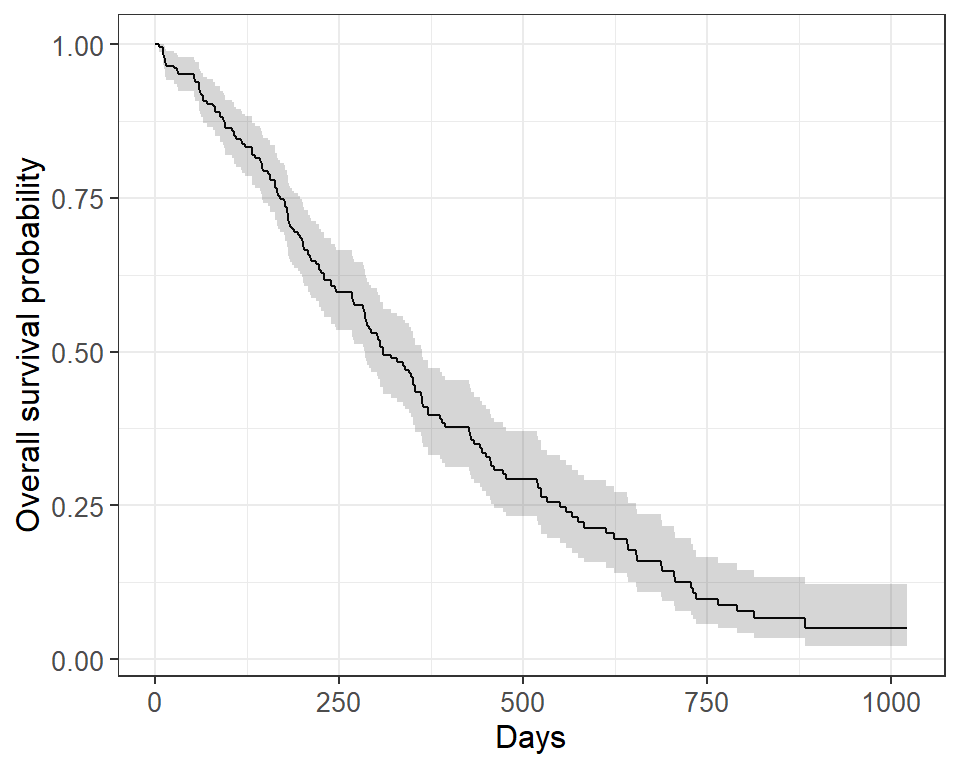
Typically we will also want to see the numbers at risk in a table below the x-axis. We can add this using add_risktable() :
survfit2(Surv(time, status) ~ 1, data = lung) %>% ggsurvfit() + labs( x = "Days", y = "Overall survival probability" ) + add_confidence_interval() + add_risktable()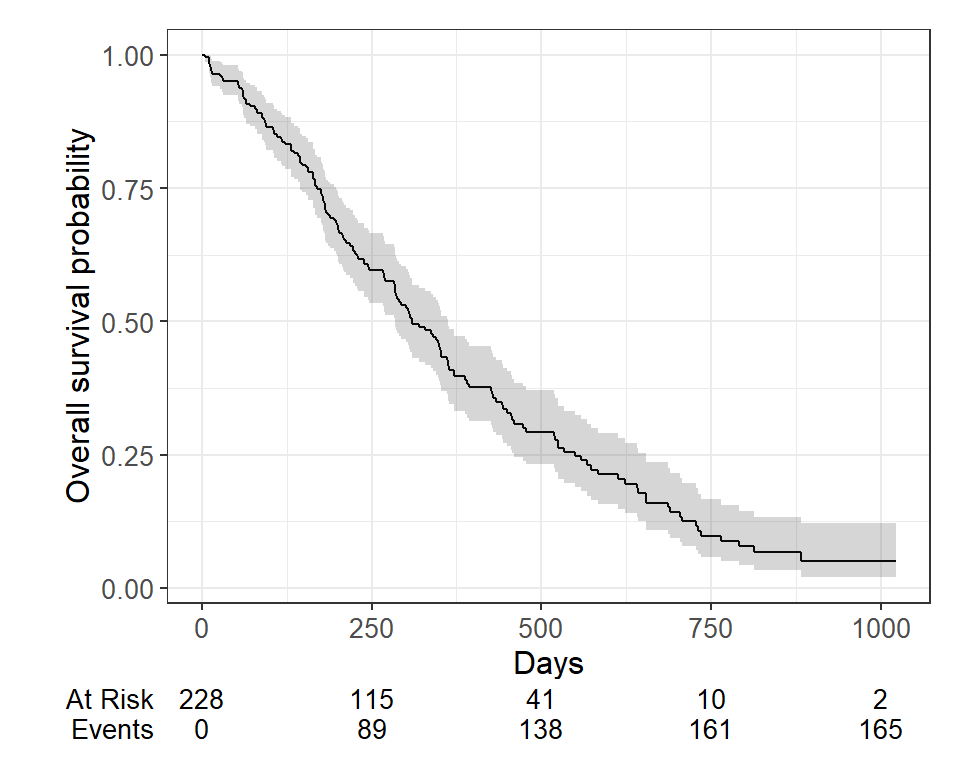
Plots can be customized using many standard options.
One quantity often of interest in a survival analysis is the probability of surviving beyond a certain number of years, \(x\) .
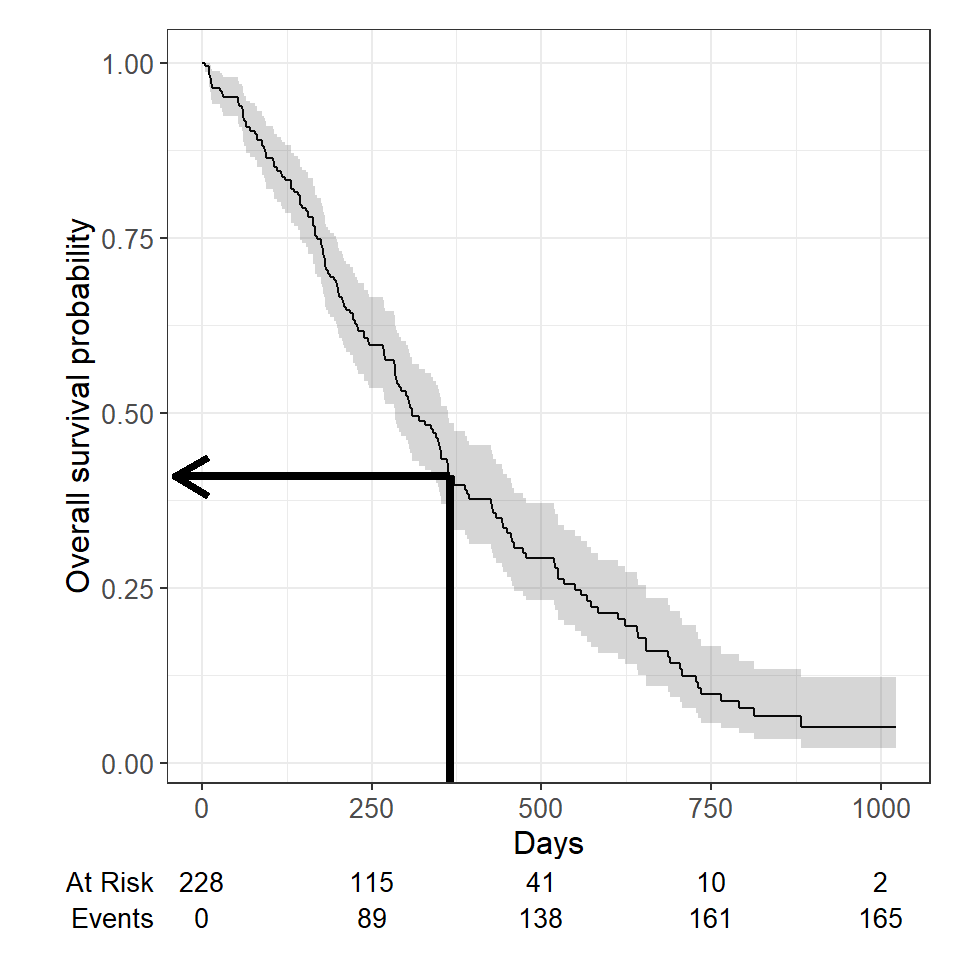
For example, to estimate the probability of surviving to \(1\) year, use summary with the times argument (Note: the time variable in the lung data is actually in days, so we need to use times = 365.25 )
summary(survfit(Surv(time, status) ~ 1, data = lung), times = 365.25)## Call: survfit(formula = Surv(time, status) ~ 1, data = lung) ## ## time n.risk n.event survival std.err lower 95% CI upper 95% CI ## 365 65 121 0.409 0.0358 0.345 0.486We find that the \(1\) -year probability of survival in this study is 41%.
The associated lower and upper bounds of the 95% confidence interval are also displayed.
The \(1\) -year survival probability is the point on the y-axis that corresponds to \(1\) year on the x-axis for the survival curve.
What happens if you use a “naive” estimate? Here “naive” means that the patients who were censored prior to 1-year are considered event-free and included in the denominator.
121 of the 228 patients in the lung data died by \(1\) year so the “naive” estimate is calculated as:
\[\Big(1 - \frac\Big) \times 100 = 47\%\] You get an incorrect estimate of the \(1\) -year probability of survival when you ignore the fact that 42 patients were censored before \(1\) year.
Recall the correct estimate of the \(1\) -year probability of survival, accounting for censoring using the Kaplan-Meier method, was 41%.
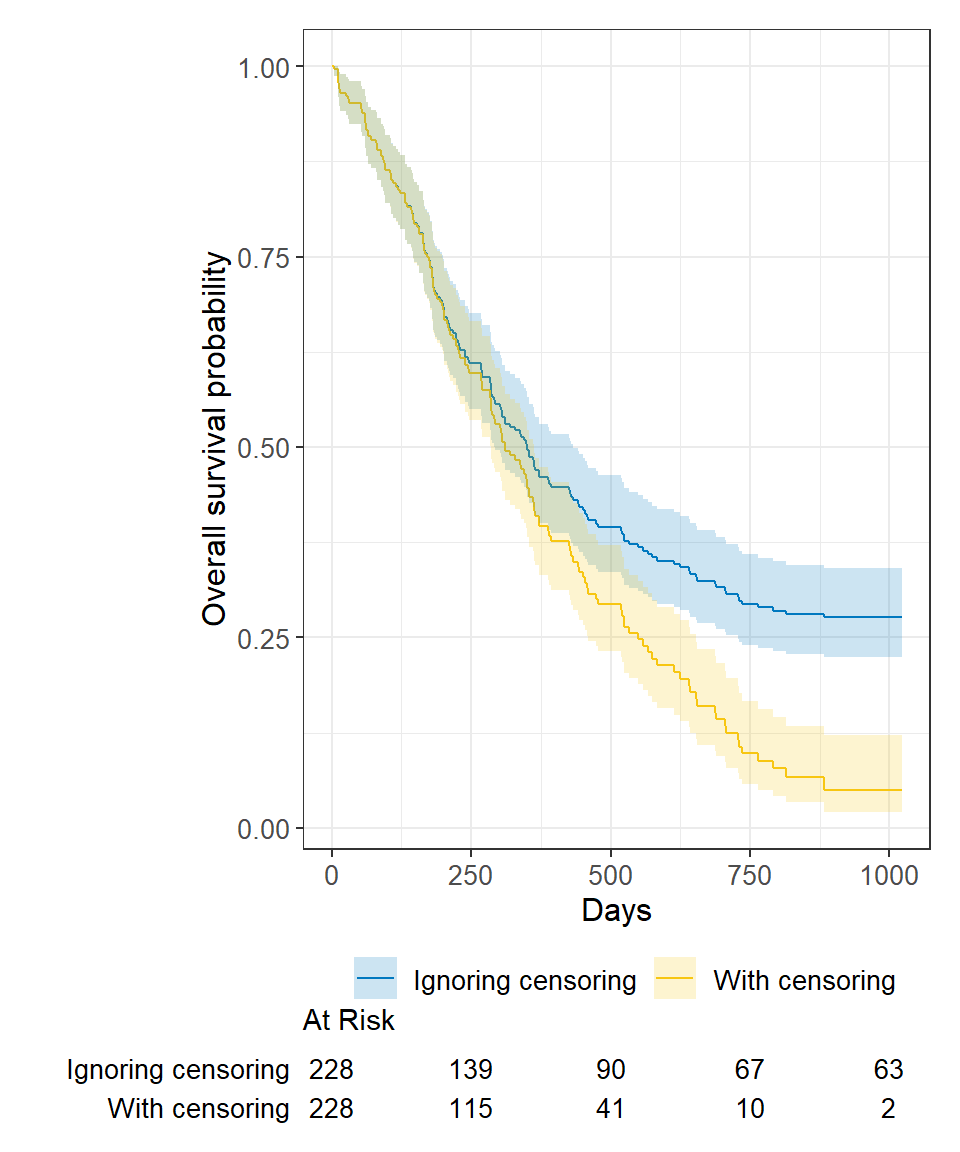
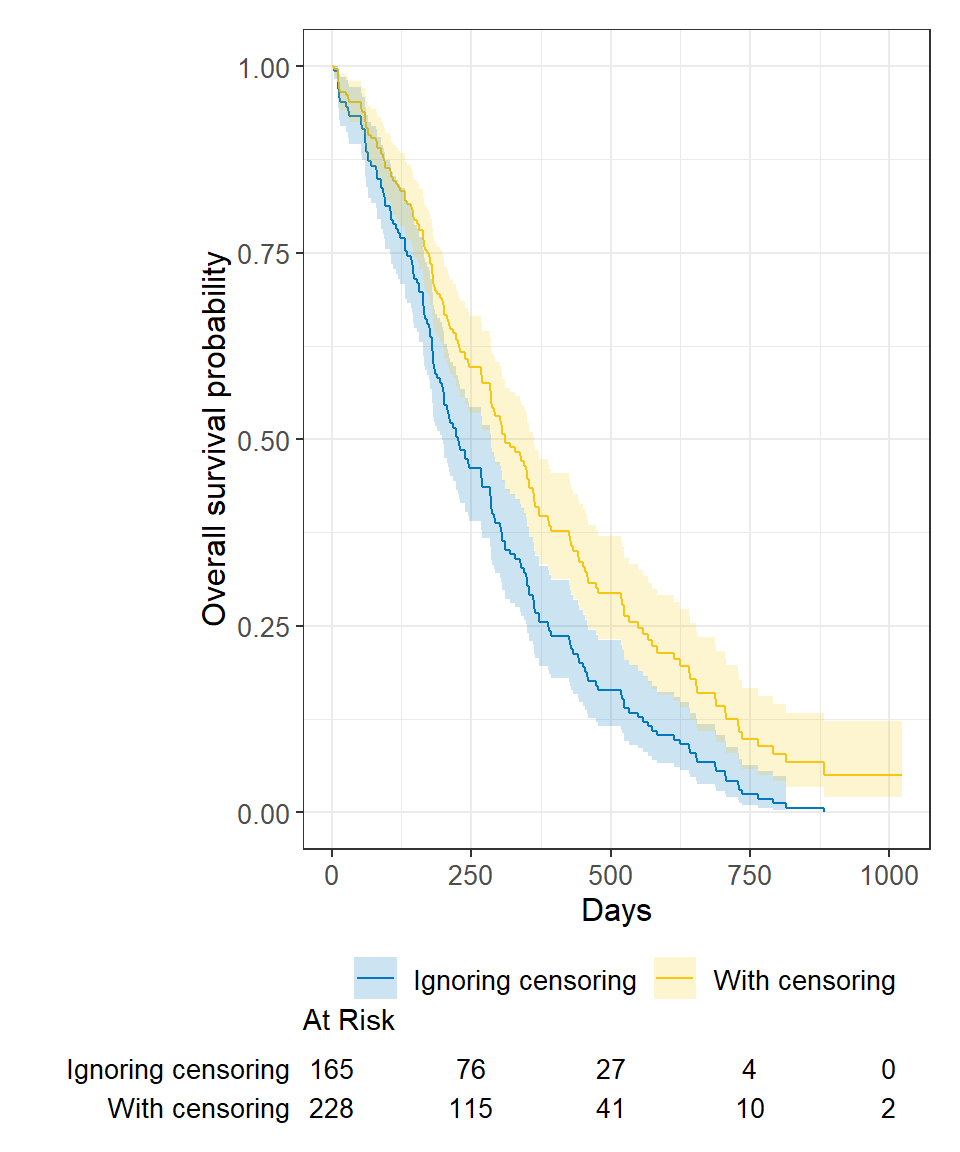
Ignoring censoring leads to an overestimate of the overall survival probability. Imagine two studies, each with 228 subjects. There are 165 deaths in each study. Censoring is ignored in one (blue line), censoring is accounted for in the other (yellow line). The censored subjects only contribute information for a portion of the follow-up time, and then fall out of the risk set, thus pulling down the cumulative probability of survival. Ignoring censoring erroneously treats patients who are censored as part of the risk set for the entire follow-up period.
We can produce nice tables of \(x\) -time survival probability estimates using the tbl_survfit() function from the package:
survfit(Surv(time, status) ~ 1, data = lung) %>% tbl_survfit( times = 365.25, label_header = "**1-year survival (95% CI)**" )| Characteristic | 1-year survival (95% CI) |
|---|---|
| Overall | 41% (34%, 49%) |
Another quantity often of interest in a survival analysis is the average survival time, which we quantify using the median. Survival times are not expected to be normally distributed so the mean is not an appropriate summary.
We can obtain the median survival directly from the survfit object:
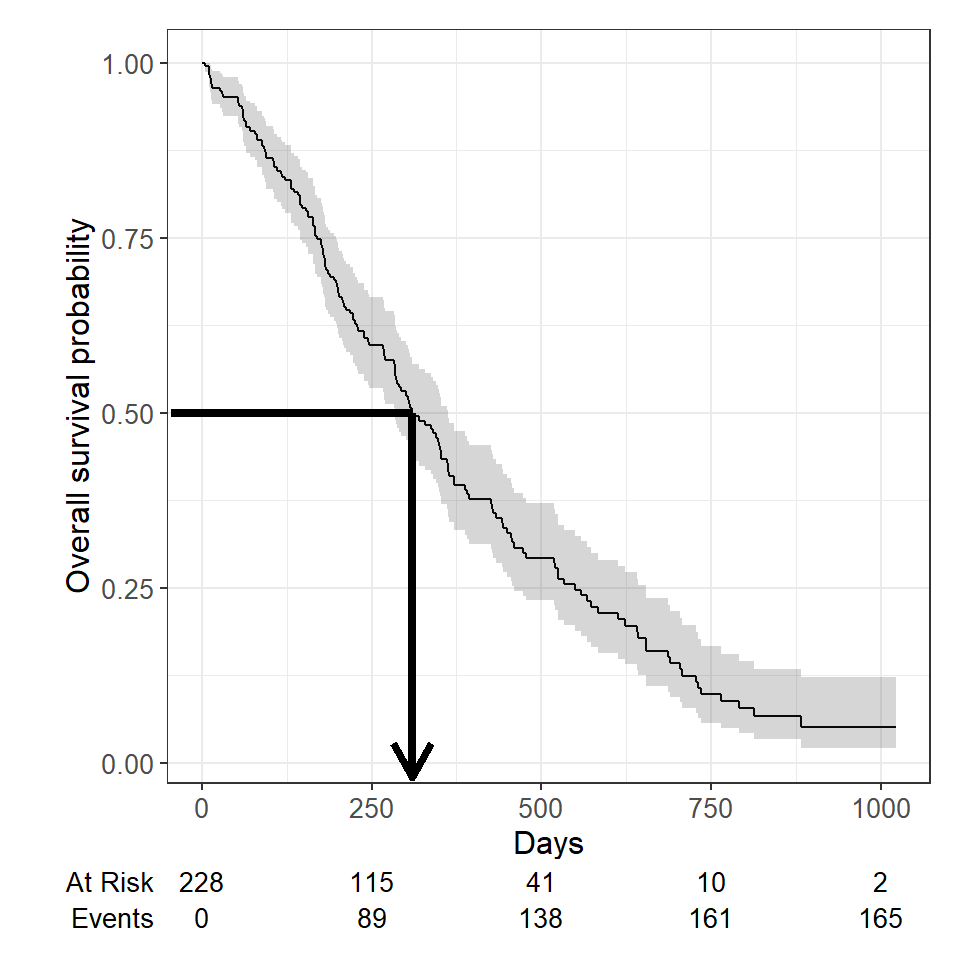
survfit(Surv(time, status) ~ 1, data = lung)## Call: survfit(formula = Surv(time, status) ~ 1, data = lung) ## ## n events median 0.95LCL 0.95UCL ## [1,] 228 165 310 285 363We see the median survival time is 310 days The lower and upper bounds of the 95% confidence interval are also displayed.
Median survival is the time corresponding to a survival probability of \(0.5\) :
What happens if you use a “naive” estimate? Here “naive” means that you exclude the censored patients from the calculation entirely to estimate median survival time among the patients who have had the event.
Summarize the median survival time among the 165 patients who died:
lung %>% filter(status == 1) %>% summarize(median_surv = median(time))## median_surv ## 1 226You get an incorrect estimate of median survival time of 226 days when you ignore the fact that censored patients also contribute follow-up time.
Recall the correct estimate of median survival time is 310 days.
Ignoring censoring will lead to an underestimate of median survival time because the follow-up time that censored patients contribute is excluded (blue line). The true survival curve accounting for censoring in the lung data is shown in yellow for comparison.
We can produce nice tables of median survival time estimates using the tbl_survfit() function from the package:
survfit(Surv(time, status) ~ 1, data = lung) %>% tbl_survfit( probs = 0.5, label_header = "**Median survival (95% CI)**" )| Characteristic | Median survival (95% CI) |
|---|---|
| Overall | 310 (285, 363) |
We can conduct between-group significance tests using a log-rank test. The log-rank test equally weights observations over the entire follow-up time and is the most common way to compare survival times between groups. There are versions that more heavily weight the early or late follow-up that could be more appropriate depending on the research question (see ?survdiff for different test options).
We get the log-rank p-value using the survdiff function. For example, we can test whether there was a difference in survival time according to sex in the lung data:
survdiff(Surv(time, status) ~ sex, data = lung)## Call: ## survdiff(formula = Surv(time, status) ~ sex, data = lung) ## ## N Observed Expected (O-E)^2/E (O-E)^2/V ## sex=1 138 112 91.6 4.55 10.3 ## sex=2 90 53 73.4 5.68 10.3 ## ## Chisq= 10.3 on 1 degrees of freedom, p= 0.001We see that there was a significant difference in overall survival according to sex in the lung data, with a p-value of p = 0.001.
We may want to quantify an effect size for a single variable, or include more than one variable into a regression model to account for the effects of multiple variables.
The Cox regression model is a semi-parametric model that can be used to fit univariable and multivariable regression models that have survival outcomes.
\[h(t|X_i) = h_0(t) \exp(\beta_1 X_ + \cdots + \beta_p X_)\]
\(h(t)\) : hazard, or the instantaneous rate at which events occur \(h_0(t)\) : underlying baseline hazard
Some key assumptions of the model:
Note: parametric regression models for survival outcomes are also available, but they won’t be addressed in this tutorial
We can fit regression models for survival data using the coxph() function from the package, which takes a Surv() object on the left hand side and has standard syntax for regression formulas in R on the right hand side.
coxph(Surv(time, status) ~ sex, data = lung)## Call: ## coxph(formula = Surv(time, status) ~ sex, data = lung) ## ## coef exp(coef) se(coef) z p ## sex -0.5310 0.5880 0.1672 -3.176 0.00149 ## ## Likelihood ratio test=10.63 on 1 df, p=0.001111 ## n= 228, number of events= 165We can obtain tables of results using the tbl_regression() function from the package, with the option to exponentiate set to TRUE to return the hazard ratio rather than the log hazard ratio:
coxph(Surv(time, status) ~ sex, data = lung) %>% tbl_regression(exp = TRUE) | Characteristic | HR 1 | 95% CI 1 | p-value |
|---|---|---|---|
| sex | 0.59 | 0.42, 0.82 | 0.001 |
| 1 HR = Hazard Ratio, CI = Confidence Interval | |||
The quantity of interest from a Cox regression model is a hazard ratio (HR). The HR represents the ratio of hazards between two groups at any particular point in time. The HR is interpreted as the instantaneous rate of occurrence of the event of interest in those who are still at risk for the event. It is not a risk, though it is commonly mis-interpreted as such. If you have a regression parameter \(\beta\) , then HR = \(\exp(\beta)\) .
A HR < 1 indicates reduced hazard of death whereas a HR >1 indicates an increased hazard of death.
So the HR = 0.59 implies that 0.59 times as many females are dying as males, at any given time. Stated differently, females have a significantly lower hazard of death than males in these data.
In Part 1 we covered using log-rank tests and Cox regression to examine associations between covariates of interest and survival outcomes. But these analyses rely on the covariate being measured at baseline, that is, before follow-up time for the event begins. What happens if you are interested in a covariate that is measured after follow-up time begins?
Example: Overall survival is measured from treatment start, and interest is in the association between complete response to treatment and survival.
Anderson et al (JCO, 1983) described why traditional methods such as log-rank tests or Cox regression are biased in favor of responders in this scenario, and proposed the landmark approach. The null hypothesis in the landmark approach is that survival from landmark does not depend on response status at landmark.
Anderson, J., Cain, K., & Gelber, R. (1983). Analysis of survival by tumor response. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 1(11), 710-9.
Some other possible covariates of interest in cancer research that may not be measured at baseline include:
Throughout this section we will use the BMT dataset from package as an example dataset. The data consist of 137 bone marrow transplant patients. Variables of interest include:
First, load the data for use in examples throughout:
# install.packages("SemiCompRisks") data(BMT, package = "SemiCompRisks")Here are the first 6 observations:
head(BMT[, c("T1", "delta1", "TA", "deltaA")])## T1 delta1 TA deltaA ## 1 2081 0 67 1 ## 2 1602 0 1602 0 ## 3 1496 0 1496 0 ## 4 1462 0 70 1 ## 5 1433 0 1433 0 ## 6 1377 0 1377 0In the BMT data, interest is in the association between acute graft versus host disease (aGVHD) and survival. But aGVHD is assessed after the transplant, which is our baseline, or start of follow-up, time.
Step 1 Select landmark time
Typically aGVHD occurs within the first 90 days following transplant, so we use a 90-day landmark.
Step 2 Subset population for those followed at least until landmark time
lm_dat % filter(T1 >= 90) We exclude 15 patients who were not followed until the landmark time of 90 days.
Note: All 15 excluded patients died before the 90 day landmark.
Step 3 Calculate follow-up time from landmark and apply traditional methods.
lm_dat % mutate( lm_T1 = T1 - 90 )survfit2(Surv(lm_T1, delta1) ~ deltaA, data = lm_dat) %>% ggsurvfit() + labs( x = "Days from 90-day landmark", y = "Overall survival probability" ) + add_risktable()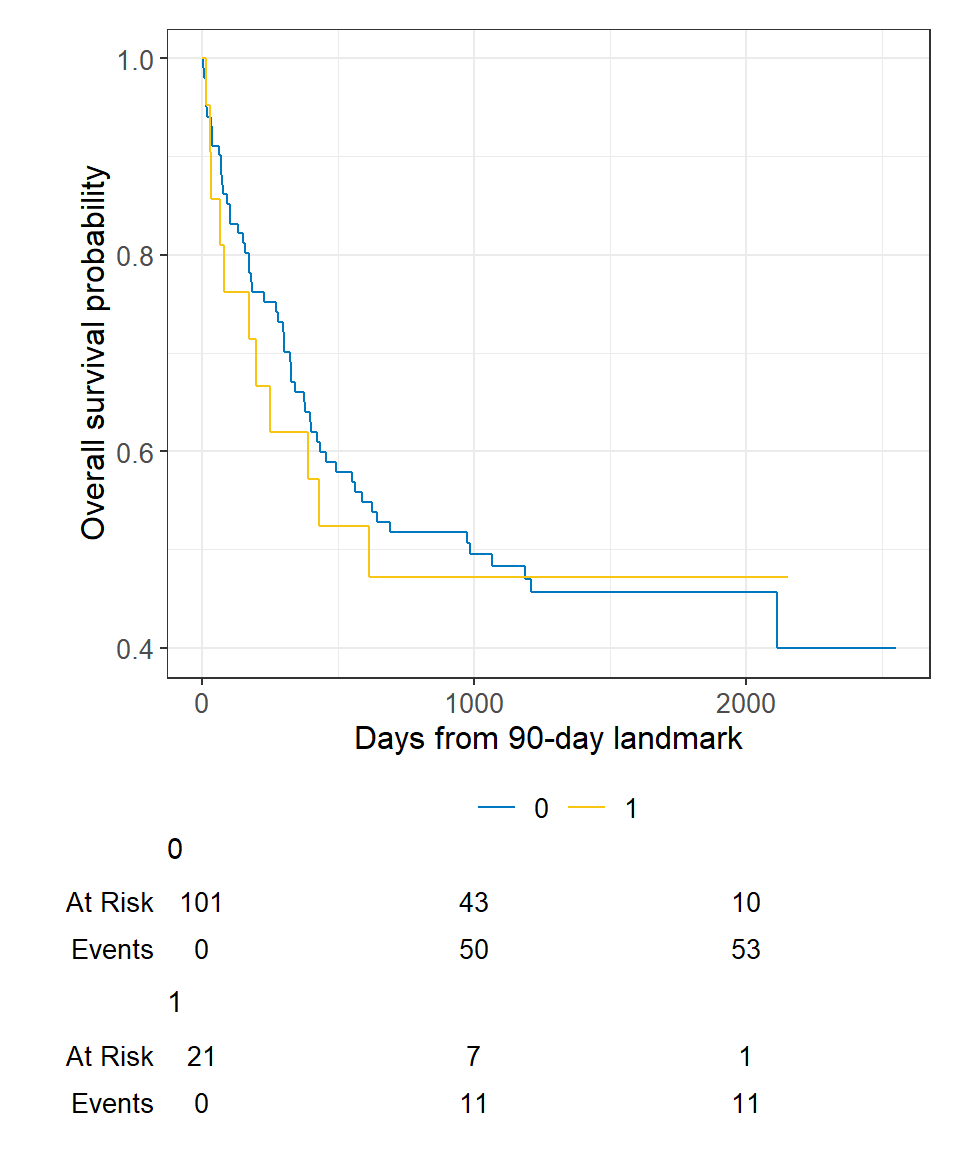
In Cox regression you can use the subset option in coxph to exclude those patients who were not followed through the landmark time, and we can view the results using the tbl_regression() function from the package:
coxph( Surv(T1, delta1) ~ deltaA, subset = T1 >= 90, data = BMT ) %>% tbl_regression(exp = TRUE)| Characteristic | HR 1 | 95% CI 1 | p-value |
|---|---|---|---|
| deltaA | 1.08 | 0.57, 2.07 | 0.8 |
| 1 HR = Hazard Ratio, CI = Confidence Interval | |||
An alternative to a landmark analysis is incorporation of a time-dependent covariate. This may be more appropriate than landmark analysis when:
There was no ID variable in the BMT data, which is needed to create the special dataset, so create an ID variable called my_id :
Use the tmerge function with the event and tdc function options to create the special dataset.
td_dat % select(my_id, T1, delta1), data2 = BMT %>% select(my_id, T1, delta1, TA, deltaA), death = event(T1, delta1), agvhd = tdc(TA) )To see what this does, let’s look at the data for the first 5 individual patients. The variables of interest in the original data looked like:
## my_id T1 delta1 TA deltaA ## 1 1 2081 0 67 1 ## 2 2 1602 0 1602 0 ## 3 3 1496 0 1496 0 ## 4 4 1462 0 70 1 ## 5 5 1433 0 1433 0The new dataset for these same patients looks like:
## my_id T1 delta1 tstart tstop death agvhd ## 1 1 2081 0 0 67 0 0 ## 2 1 2081 0 67 2081 0 1 ## 3 2 1602 0 0 1602 0 0 ## 4 3 1496 0 0 1496 0 0 ## 5 4 1462 0 0 70 0 0 ## 6 4 1462 0 70 1462 0 1 ## 7 5 1433 0 0 1433 0 0We see that patients 1 and 4 now have multiple rows of data because they both developed aGVHD at some point in time after baseline.
Now we can analyze this time-dependent covariate as usual using Cox regression with coxph and an alteration to our use of Surv to include arguments to both time and time2 :
coxph( Surv(time = tstart, time2 = tstop, event = death) ~ agvhd, data = td_dat ) %>% tbl_regression(exp = TRUE)| Characteristic | HR 1 | 95% CI 1 | p-value |
|---|---|---|---|
| agvhd | 1.40 | 0.81, 2.43 | 0.2 |
| 1 HR = Hazard Ratio, CI = Confidence Interval | |||
We find that acute graft versus host disease is not significantly associated with death using either landmark analysis or a time-dependent covariate.
Often one will want to use landmark analysis for visualization of a single covariate, and Cox regression with a time-dependent covariate for univariable and multivariable modeling.
Competing risks analyses may be used when subjects have multiple possible events in a time-to-event setting.
All or some of these (among others) may be possible events in any given study.
The fundamental problem that may lead to the need for specialized statistical methods is unobserved dependence among the various event times. For example, one can imagine that patients who recur are more likely to die, and therefore times to recurrence and times to death would not be independent events.
There are two approaches to analysis in the presence of multiple potential outcomes:
Each of these approaches may only illuminate one important aspect of the data while possibly obscuring others, and the chosen approach should depend on the question of interest.
When the events are independent (almost never true), cause-specific hazards is unbiased. When the events are dependent, a variety of results can be obtained depending on the setting.
Cumulative incidence using 1 minus the Kaplan-Meier estimate is always >= cumulative incidence using competing risks methods, so can only lead to an overestimate of the cumulative incidence, though the amount of overestimation depends on event rates and dependence among events.
To establish that a covariate is indeed acting on the event of interest, cause-specific hazards may be preferred for treatment or prognostic marker effect testing. To establish overall benefit, subdistribution hazards may be preferred for building prognostic nomograms or considering health economic effects to get a better sense of the influence of treatment and other covariates on an absolute scale.
Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301-8.
Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007 Jan 15;13(2 Pt 1):559-65.
Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229-35.
Austin, P., & Fine, J. (2017). Practical recommendations for reporting Fine‐Gray model analyses for competing risk data. Statistics in Medicine, 36(27), 4391-4400.
We can obtain the cause-specific hazard of a given event using the methods introduced in the previous section, where the event of interest counts as an event and any competing events are censored at the date of the competing even.
The rest of this section will focus on methods for subdistribtuion hazards.
The primary package we will use for competing risks analysis is the package.
We will use the Melanoma data from the package to illustrate these concepts. It contains variables:
# install.packages("MASS") data(Melanoma, package = "MASS")The status variable in these data are coded in a non-standard way. Let’s recode to avoid confusion:
Melanoma % mutate( status = as.factor(recode(status, `2` = 0, `1` = 1, `3` = 2)) )Here are the first 6 patients:
head(Melanoma)## time status sex age year thickness ulcer ## 1 10 2 1 76 1972 6.76 1 ## 2 30 2 1 56 1968 0.65 0 ## 3 35 0 1 41 1977 1.34 0 ## 4 99 2 0 71 1968 2.90 0 ## 5 185 1 1 52 1965 12.08 1 ## 6 204 1 1 28 1971 4.84 1A non-parametric estimate of the cumulative incidence of the event of interest. At any point in time, the sum of the cumulative incidence of each event is equal to the total cumulative incidence of any event (not true in the cause-specific setting). Gray’s test is a modified Chi-squared test used to compare 2 or more groups.
Estimate the cumulative incidence in the context of competing risks using the cuminc function from the package. By default this requires the status to be a factor variable with censored patients coded as 0.
cuminc(Surv(time, status) ~ 1, data = Melanoma)## ## time n.risk estimate std.error 95% CI ## 1,000 171 0.127 0.023 0.086, 0.177 ## 2,000 103 0.230 0.030 0.174, 0.291 ## 3,000 54 0.310 0.037 0.239, 0.383 ## 4,000 13 0.339 0.041 0.260, 0.419 ## 5,000 1 0.339 0.041 0.260, 0.419 ## ## time n.risk estimate std.error 95% CI ## 1,000 171 0.034 0.013 0.015, 0.066 ## 2,000 103 0.050 0.016 0.026, 0.087 ## 3,000 54 0.058 0.017 0.030, 0.099 ## 4,000 13 0.106 0.032 0.053, 0.179 ## 5,000 1 0.106 0.032 0.053, 0.179We can use the ggcuminc() function from the package to plot the cumulative incidence. By default it plots the first event type only. So the following plot shows the cumulative incidence of death from melanoma:
cuminc(Surv(time, status) ~ 1, data = Melanoma) %>% ggcuminc() + labs( x = "Days" ) + add_confidence_interval() + add_risktable()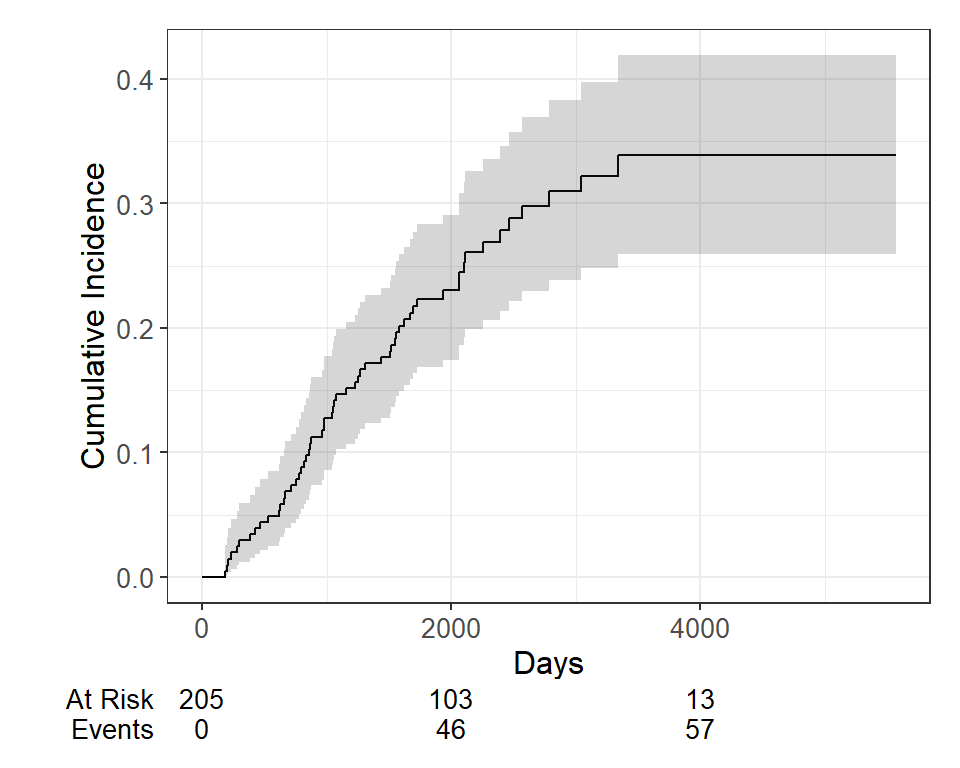
If we want to include both event types, specify the outcomes in the ggcuminc(outcome=) argument:
cuminc(Surv(time, status) ~ 1, data = Melanoma) %>% ggcuminc(outcome = c("1", "2")) + ylim(c(0, 1)) + labs( x = "Days" )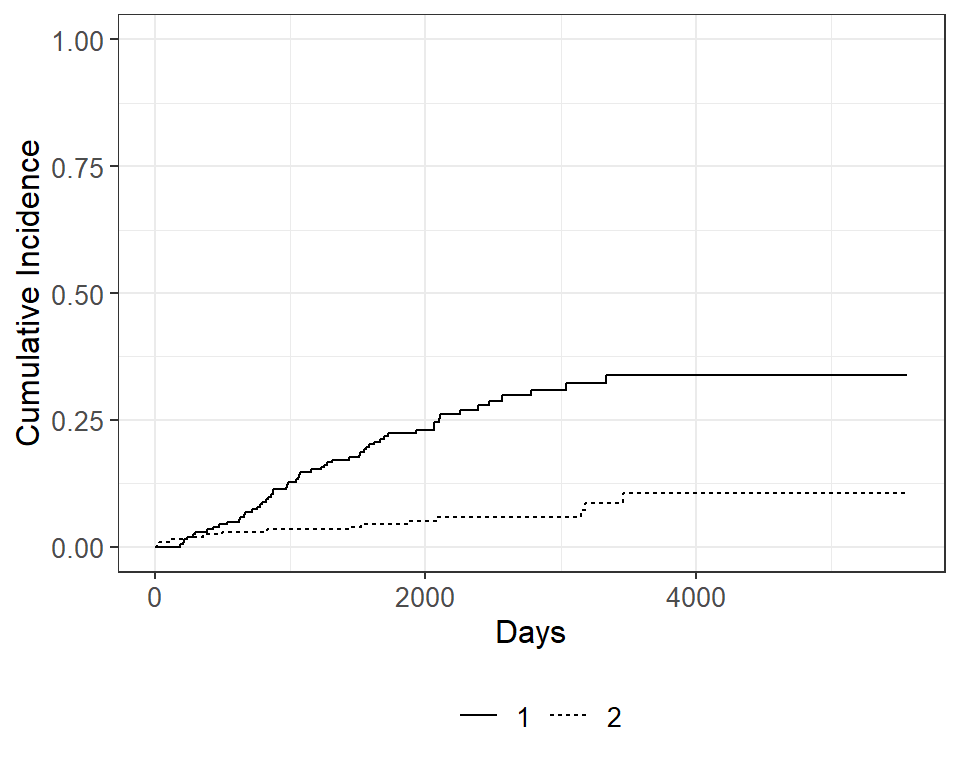
Now let’s say we wanted to examine death from melanoma or other causes in the Melanoma data, according to ulcer , the presence or absence of ulceration. We can estimate the cumulative incidence at various times by group and display that in a table using the tbl_cuminc() function from the package, and add Gray’s test to test for a difference between groups over the entire follow-up period using the add_p() function.
cuminc(Surv(time, status) ~ ulcer, data = Melanoma) %>% tbl_cuminc( times = 1826.25, label_header = "**-year cuminc**") %>% add_p()| Characteristic | 5-year cuminc | p-value 1 |
|---|---|---|
| ulcer | ||
| 1 Gray’s Test | ||
Then we can see the plot of death due to melanoma, according to ulceration status, as before using ggcuminc() from the package:
cuminc(Surv(time, status) ~ ulcer, data = Melanoma) %>% ggcuminc() + labs( x = "Days" ) + add_confidence_interval() + add_risktable()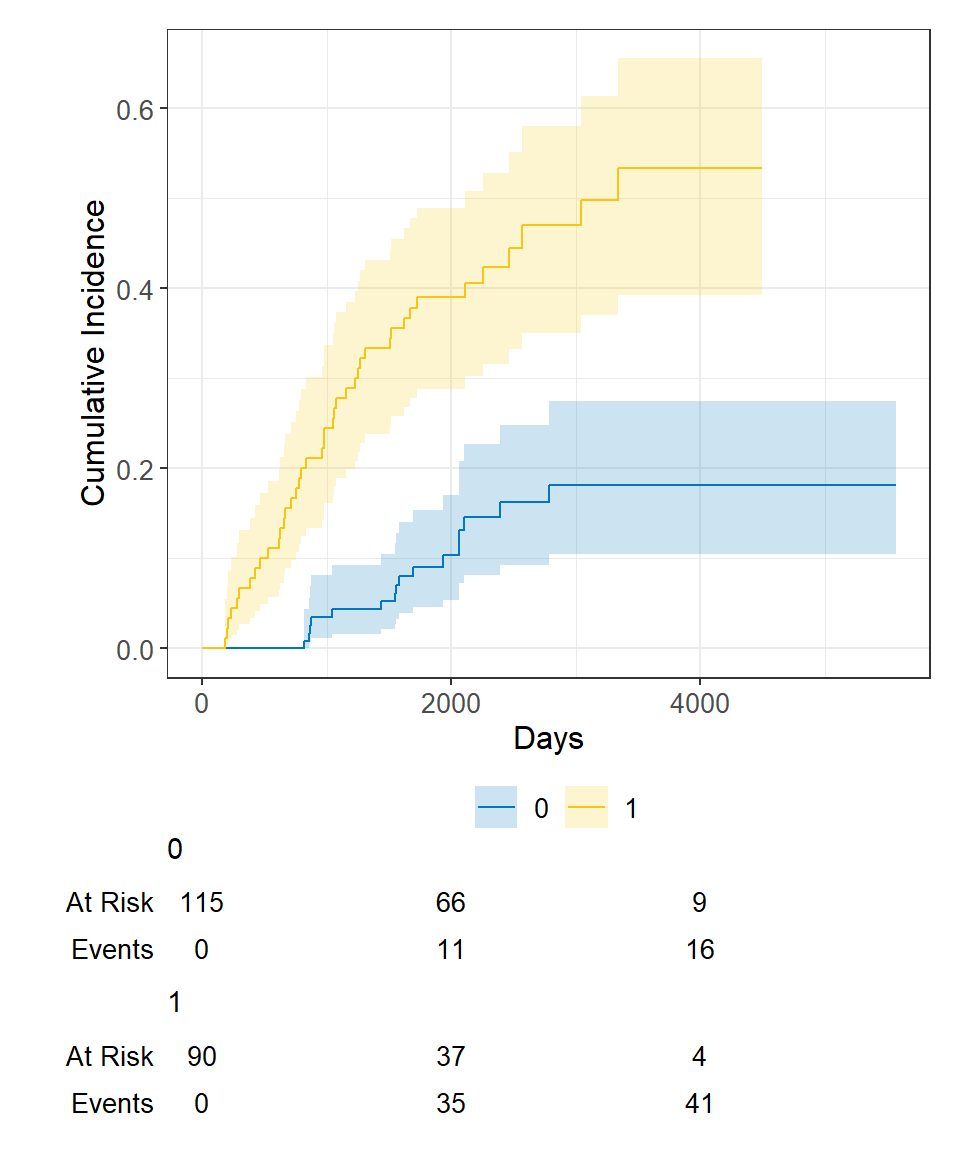
There are two approaches to competing risks regression:
Let’s say we’re interested in looking at the effect of age and sex on death from melanoma, with death from other causes as a competing event.
The crr() function from the package will estimate the subdistribution hazards.
crr(Surv(time, status) ~ sex + age, data = Melanoma)## ## Variable Coef SE HR 95% CI p-value ## sex 0.588 0.272 1.80 1.06, 3.07 0.030 ## age 0.013 0.009 1.01 0.99, 1.03 0.18And we can generate tables of formatted results using the tbl_regression() function from the package, with the option exp = TRUE to obtain the hazard ratio estimates:
crr(Surv(time, status) ~ sex + age, data = Melanoma) %>% tbl_regression(exp = TRUE)| Characteristic | HR 1 | 95% CI 1 | p-value |
|---|---|---|---|
| sex | 1.80 | 1.06, 3.07 | 0.030 |
| age | 1.01 | 0.99, 1.03 | 0.2 |
| 1 HR = Hazard Ratio, CI = Confidence Interval | |||
We see that male sex (recall that 1=male, 0=female in these data) is significantly associated with increased hazard of death due to melanoma, whereas age was not significantly associated with death due to melanoma.
Alternatively, if we wanted to use the cause-specific hazards regression approach, we first need to censor all subjects who didn’t have the event of interest, in this case death from melanoma, and then use coxph as before. So patients who died from other causes are now censored for the cause-specific hazard approach to competing risks. Again we generate a table of formatted results using the tbl_regression() function from the package:
coxph( Surv(time, ifelse(status == 1, 1, 0)) ~ sex + age, data = Melanoma ) %>% tbl_regression(exp = TRUE)| Characteristic | HR 1 | 95% CI 1 | p-value |
|---|---|---|---|
| sex | 1.82 | 1.08, 3.07 | 0.025 |
| age | 1.02 | 1.00, 1.03 | 0.056 |
| 1 HR = Hazard Ratio, CI = Confidence Interval | |||
And in this case we obtain similar results using the two approaches to competing risks regression.
So far we’ve covered:
In this section I’ll include a variety of bits and pieces of things that may come up and be handy to know:
One assumption of the Cox proportional hazards regression model is that the hazards are proportional at each point in time throughout follow-up. The cox.zph() function from the package allows us to check this assumption. It results in two main things:
mv_fit ## chisq df p ## sex 2.608 1 0.11 ## age 0.209 1 0.65 ## GLOBAL 2.771 2 0.25plot(cz)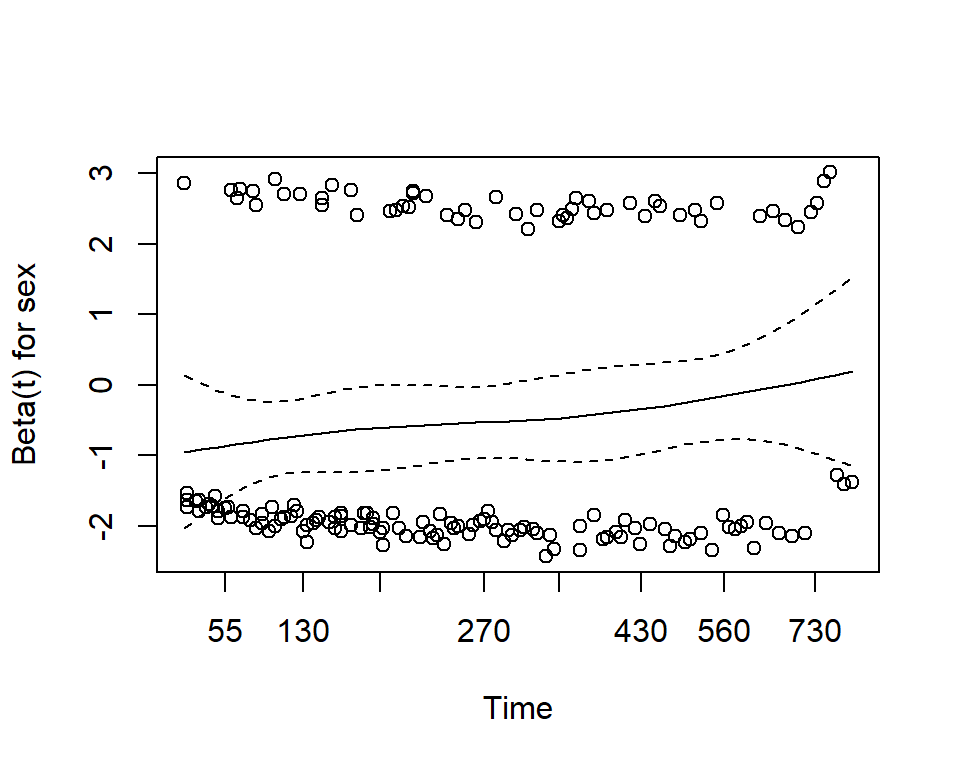
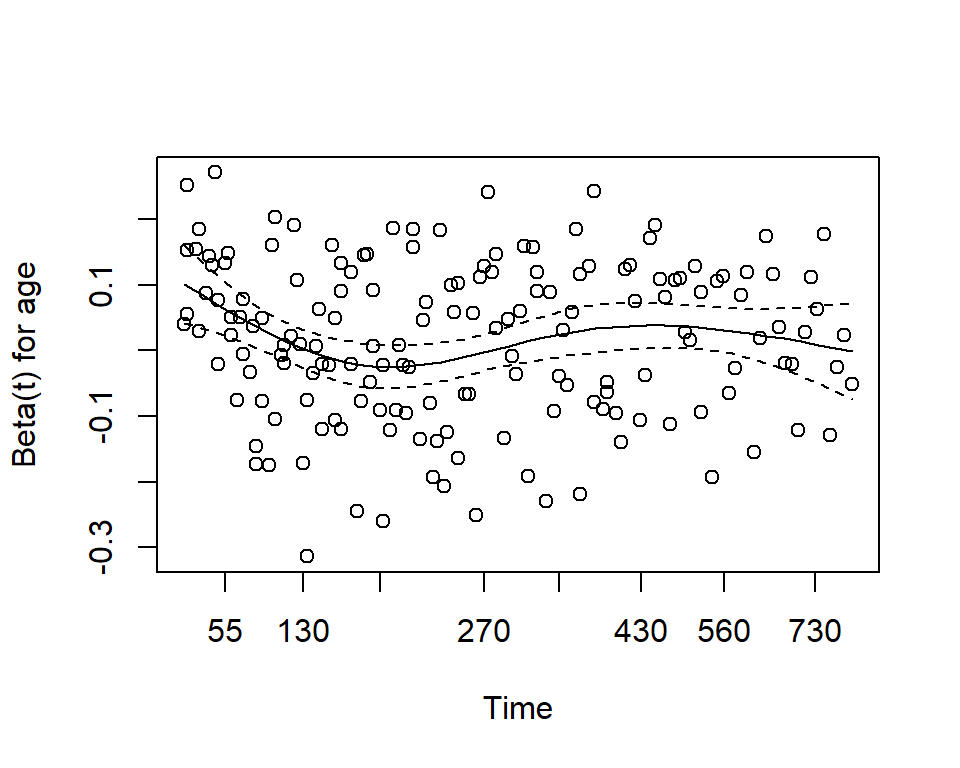
Here we see that with p-values >0.05, we do not reject the null hypothesis, and conclude that the proportional hazards assumption is satisfied for each individual covariate, and also for the model overall.
Sometimes you will want to visualize a survival estimate according to a continuous variable. The sm.survival function from the sm package allows you to do this for a quantile of the distribution of survival data. The default quantile is p = 0.5 for median survival.
# install.packages("sm") library(sm) sm.options( list( xlab = "Age (years)", ylab = "Median time to death (years)") ) sm.survival( x = lung$age, y = lung$time, status = lung$status, h = sd(lung$age) / nrow(lung)^(-1/4) )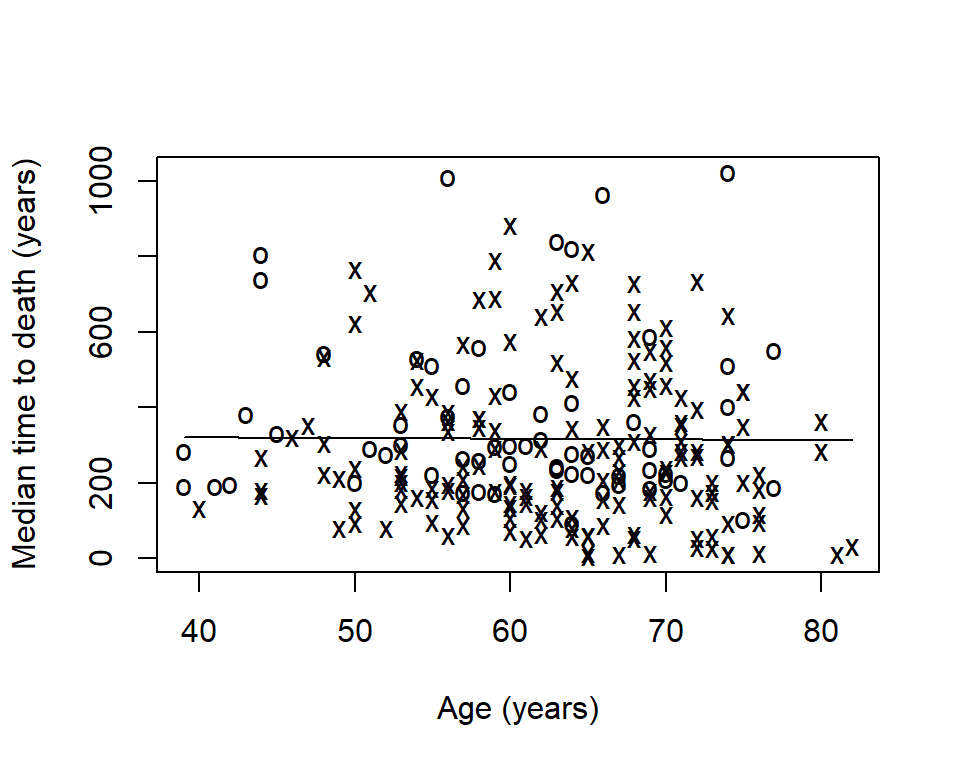
The option h is the smoothing parameter. This should be related to the standard deviation of the continuous covariate, \(x\) . Suggested to start with \(\frac>\) then reduce by \(1/2\) , \(1/4\) , etc to get a good amount of smoothing. The previous plot was too smooth so let’s reduce it by \(1/6\) :
sm.survival( x = lung$age, y = lung$time, status = lung$status, h = (1/6) * sd(lung$age) / nrow(lung)^(-1/4) )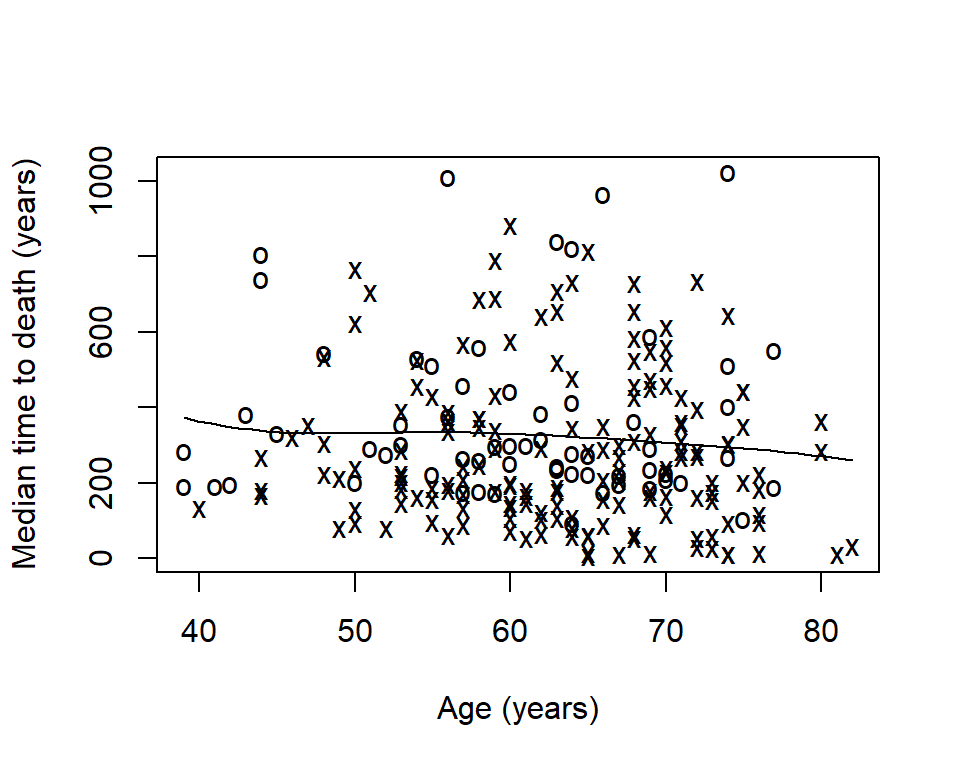
Now we can see that median time to death decreases slightly as age increases.
Sometimes it is of interest to generate survival estimates among a group of patients who have already survived for some length of time.
Zabor, E., Gonen, M., Chapman, P., & Panageas, K. (2013). Dynamic prognostication using conditional survival estimates. Cancer, 119(20), 3589-3592.
We can use the conditional_surv_est() function from the package to get estimates and 95% confidence intervals. Let’s condition on survival to 6-months
fit1 % mutate(months = round(prob_times / 30.4)) %>% select(months, everything()) %>% kable()| months | cs_est | cs_lci | cs_uci |
|---|---|---|---|
| 12 | 0.58 | 0.49 | 0.66 |
| 18 | 0.36 | 0.27 | 0.45 |
| 24 | 0.16 | 0.10 | 0.25 |
Recall that our initial \(1\) -year survival estimate was 0.41. We see that for patients who have already survived 12 months this increases to 0.58.
We can also visualize conditional survival data based on different lengths of time survived using the condKMggplot() function from the package:
gg_conditional_surv( basekm = fit1, at = prob_times, main = "Conditional survival in lung data", xlab = "Days" ) + labs(color = "Conditional time")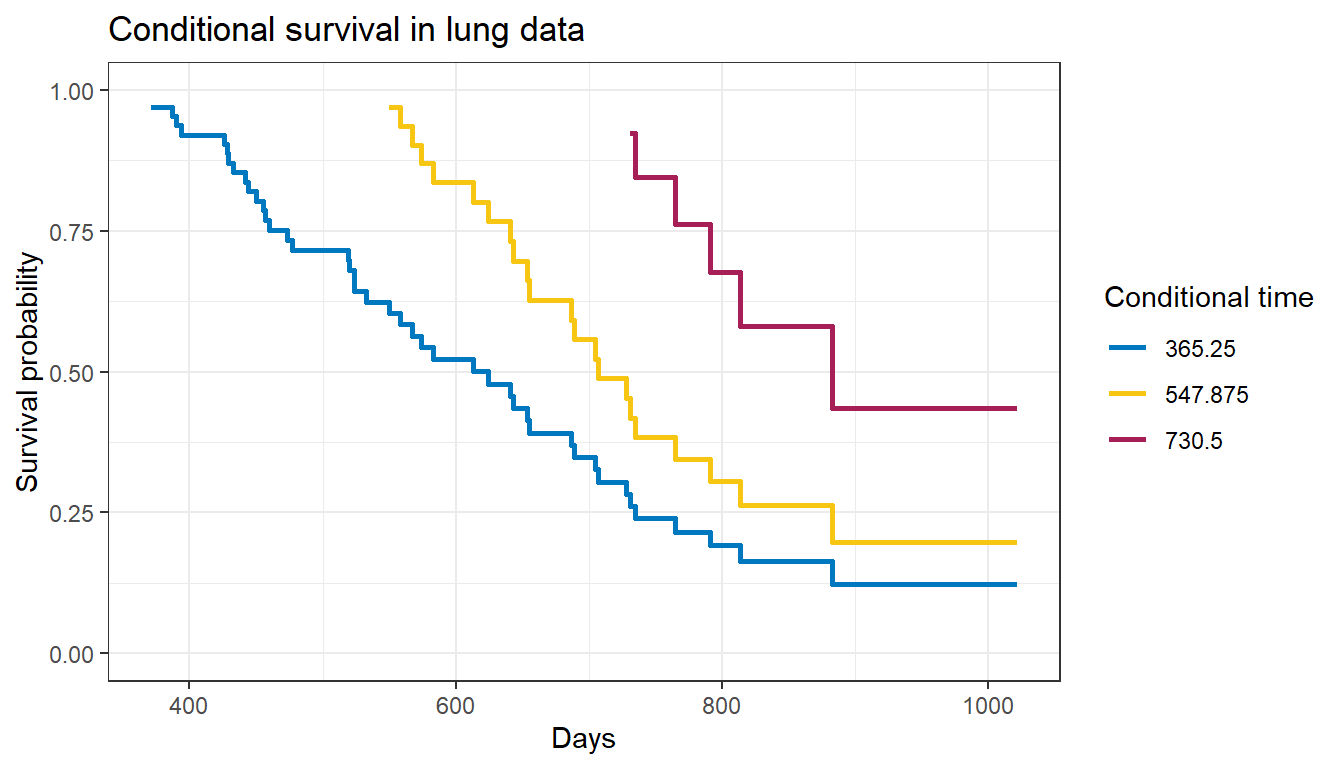
The resulting plot has one survival curve for each time on which we condition. In this case the first line is the overall survival curve since it is conditioning on time 0.